Zeroth Law of Thermodynamics is a law based on thermal equilibrium.
Thermal Equilibrium
When two bodies are in contact with each other, heat flows from hot body to cold body. And then after some time, they attain a common temperature. At this condition, the bodies are said to be in thermal equilibrium state. Thermal equilibrium does not mean that heat stops flowing from one body to another body. At this state, heat flowing from a body is equal to the heat flowing into the body.
Zeroth Law of Thermodynamics
Suppose there are three systems; $A$, $B$ and $C$ surrounded by insulating walls. Consider $B$ and $C$ are separated by heat insulating walls or adiabatic walls while $A$ is separated from $B$ and $C$ by heat conducting walls or diathermic walls.
Now, $A$ and $B$ will be in thermal equilibrium state and $A$ and $C$ will also be in thermal equilibrium state. Since, there is no change in temperature in $B$ and $C$, $B$ will be in thermal equilibrium with $C$. This is Zeroth law of thermodynamics.
Hence, Zeroth law of thermodynamics states that, “If two systems are in thermal equilibrium with a third system then the two systems themselves must be in thermal equilibrium with each other.”
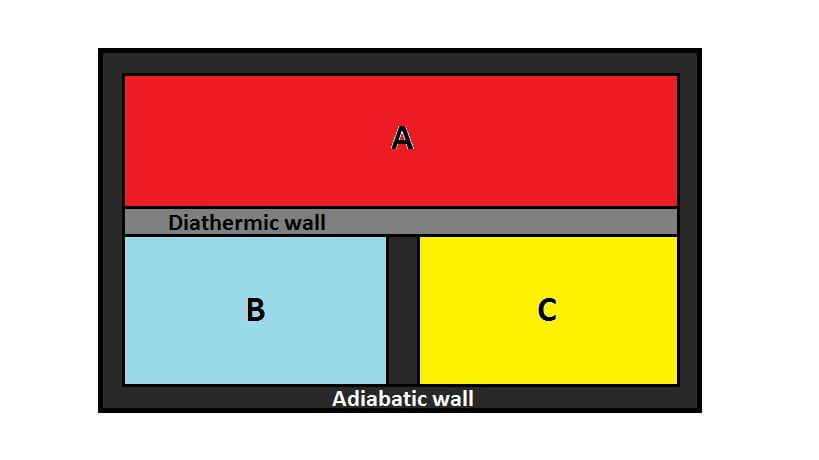
The physical quantity that determines whether $B$ is in thermal equilibrium with $C$ or not is temperature.
Thus, Zeroth law of thermodynamics may also be stated as “there exists a scalar quantity called temperature, which is a property of all thermodynamics systems, such that equality of temperature is the only condition for the thermodynamics systems to be in thermal equilibrium.”
